
| 
|

 EPSOM AND EWELL WELLS - Chapter 12
EPSOM AND EWELL WELLS - Chapter 12The Hydrogeology and Geochemistry of Epsom Wells
Dr Bruce E Osborne
Symond's and Livingstone's Well.
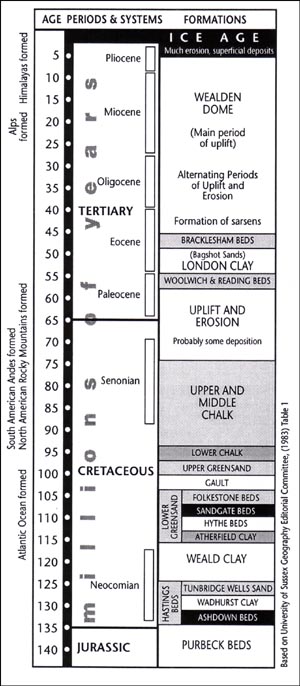
12.1. Geological table showing the sequence of sedimentary beds.[9]
Modern day scientific understanding gives new insights into the problems of the past. Our greater appreciation of the geological, chemical and hydrological causes of Epsom Salts mineralisation, shed new light on the problems encountered by Livingstone at his New Wells. Before doing this it is appropriate to consider background detail relating to Epsom salts in the eighteenth century.
The year 1735 saw the publication of A Dissertation on the Contents of Mineral Springs particularly those of Scarborough. The author noted that the salts in the best wells were trifling compared with the loads sold in shops, (Epsom waters yielded no more than four or five drams to the gallon) and that the salts were prepared in England and Germany from the Bittern or bitter liquor that remains in the preparation of common salt.[1]
Epsom appears again in the 1760/9 editions of Russell's Dissertation on Sea Water; the author was probably Russell but published posthumously; he noted that Epsom water was clear but if left in a vessel for some weeks would stink. A gallon of water would yield between 1 and 1.5 ozs. according to the season. Later he noted that the usual prescribed quantity was two thirds of a pint in summer and half a pint in winter. The salt crystals comprised rectangular prisms with parallelogram planes. Half an ounce of salt was appropriate to induce gentle purging when taken. Some took from an ounce to 10 drams in up to 4 pints of water, with a dram of mace for the same purpose, and worked it off with a posset-drink. The writer observed that it could conveniently be added to chalybeate water like Tunbridge. Poor people once used the water for washing sores to good effect.[2]
The legacy of Epsom is adequately summed up in 1784.
"EPSOM SALT. This salt has been found in considerable proportions in the purging mineral waters of Epsom in Surrey; from whence it still preserves the name of Epsom salt; though it may be procured in much larger quantities from the bitter liquor which remains after the crystalization of common salt.
Epsom, or, as it is called in the shops, the bitter cathartic salt, is composed of the vitriolic acid and the earth of magnesia: and as the vitriolic acid has a more powerful attraction to a fixed alkali, than to any of the absorbent earths, it furnishes us with an easy test by which we may distinguish Epsom from Glauber salt, when they are in state of solution. If we add a few drops of the ley of tartar to a solution of Glauber's salt in distilled water, no change is observable; but when we add these to a solution of Epsom salt, a precipitation of the earth of magnesia immediately takes place...." [3]
Ancient salt lakes, usually formed by the evaporation of sea water, when geological strata is being laid down, results in saline originated minerals being present in the sedimentary rock. Gypsum is such an evaporite mineral (CaSO4.2H2O). It is found in clays and limestones, sometimes associated with sulphur. Sodium chloride (NaCl) is widely exploited as a product of marine evaporation, both as a geological deposit and by solar evaporation in hot climates. Characteristic geological deposits, which often are changed by the action of ground water, include calcium, magnesium and sulphate ions. The temperature and ion concentration determines the actual substances deposited. Such deposits are usually laid down in reverse order of solubility, that is with the least soluble deposited first. Epsom Salts (MgSO4,7H2O) can occur as a secondary product resulting from the transformation of the products of the original evaporite sequence. The crystals are orthorhombic. In a dry environment, hexahydrite (MgSO4.6H2O) can be formed while in Stassfurt, Russia a massive variety exists, probably as a result of hydration called Reichardite (MgSO4.H2O).[4]
Epsom Salt (Magnesium Sulphate), also described as Epsomite, and the sequence of saline deposition in which the salts occur is given by Clarke (1924). It is noted that Epsomite so deposited is commercially valuable.[5] Under natural conditions calcium carbonate is the first solid to separate on evaporation of seawater followed by calcium sulphate. Only when the evaporating body is reduced to 1.54 percent of its original volume do magnesium salts crystalise. The figure may vary according to specific conditions. Natural Epsom Salts is relatively rare because the required conditions are rarely attained.[6]
Epsom lies at the junction of the London Clay to the northwest, this explains why Clay Hill was the old name for West Hill with the Chalk to the southeast. At the junction, the Tertiary strata of the Woolwich and Reading Beds and the Thanet Sands surface adjacent to the London Clay and Chalk respectively. This gives a clearly defined NE/SW surface boundary that extends for many miles. The chalk dips below the Tertiary strata to the north to give an artesian aquifer beneath the London basin.
The Woolwich and Reading Beds particularly, and the Thanet Sands possibly, contain the sulphates and magnesium necessary for the creation of Epsom Salts together with iron deposits which give a chalybeate water. These Tertiary Beds are the aquifer for ground water containing Epsom Salts. As a result of the artesian pressure of the ground water in the underlying chalk, it seeps to the surface through the Tertiary strata up to one kilometre north of the point where the chalk dips below the Tertiaries. This occurs as a result of discontinuities and fissures in the Tertiary strata and gives rise to ponds and springs.[7] Whitaker (1912) comments on the volume of water obtainable from the Thanet Sands at depth and ascribes this to the water from the underlying chalk forcing its way upward through the clayey bed at the base of the sands.[8] Such passage is not uniform and this clarifies why Epsom mineralised water sources are haphazard. Ponds and springs, as surface features, would likely be charged with Epsom Salts and this explains the original occurrence of the salts in a pond on the common c.1600 reported by Brayley, paraphrasing Fuller.
Any attempt to dig a well in the Tertiary strata would also be likely to produce water charged with Epsom Salts, although the volume available would be subject to the local aquifer conditions. This is confirmed by the well digging account of Mr Symonds, reported by Benjamin Allen of 1699, details of which are considered shortly. The selenite bed was seen to overlay a black earth with iron. This is typical of the Tertiary strata. If a well were dug directly in the chalk below however, the water yielded would be charged with calcium carbonate and would not have the characteristics resulting from the raised aquifer in the Woolwich and Reading Beds and the Thanet Sands. 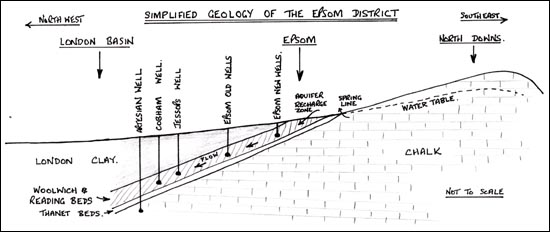
12.2: Simplified Geology of the Epsom District, showing how the artesian wells of the London basin penetrate the chalk aquifer whereas the saline wells of Epsom only reach the Woolwich and Reading Beds.
This sequence with its hydrogeological characteristics, are confirmed by Stamp who observed the Bullhead bed at the base of the water bearing Thanet sands. These in turn overlay the chalk aquifer that is sub-artesian.[10]
The stratigraphy of a trial borehole made at Epsom waterworks in 1903 and reported by Messrs. W V Graham and H Dewy, further illustrates the sequence of beds.[11] 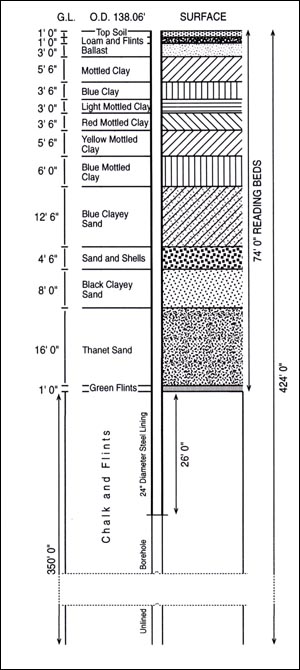
Sand and flints 8 (feet)
Reading Beds: Mottled clay 4
Red clay 5
Blue clay 2
Brown clay 3
Green sand 11
Sand and shell (ostrea bellovacina) 4
Black sand 7
Thanet Sand: Grey sand 20
Sand and flints -> chalk and flints 1
Comparisons between the 1903 sequence and a 1960 sequence given below are remarkably consistent and give a clear indication of the sub-strata in the Epsom area. Such a sequence only applied where the Tertiary strata overlays the chalk. This would be northwest of the surface juncture of the chalk and Tertiaries which runs through Epsom; the further northwest, the deeper the Woolwich and Reading Beds beneath the London Clay.
12.3. Borehole sequence at East Street Waterworks 1960 by Epsom and Ewell Water Department, supplied by Bourne Hall Museum.
The question which now arises is where was Livingstone's well? If it was located in or penetrated directly into the chalk strata, it would not have contained Epsom Salts, in which case he would have had to add the salts by artificial means. If the well was dug in the Tertiary sequence he would likely have been more fortunate, although the strength of the well would have been variable according to local geological conditions.
An important geochemical report was fortunately recorded for posterity in 1699. Benjamin Allen in The Natural History of Chalybeate and Purging Waters of England gave an account of the celebrated wells at Epsom. The present author's comments are in square brackets.
"Epsom Water was the first, of the Purging Kind, discover'd in England, viz. 1630, or soon after. The Hill is a Clay of a brown Colour and reddish; and where the Wells are, more gray.
The Well is about twelve Foot deep; the Earth where the Spring is, afforded the Selenites [a transparent and beautiful variety of Gypsum/calcium sulphate]plentifully, at a private Well there were Columns, the Sides and Superficies of which were inequi-lateral Paralelograms posited with their Edges downward, and their Ends meeting in the Centre: In a Well a few Feet distant, and at the publick Well, they were Rhomboid. [3 wells: i) the private well was Mr Symonds, ii) another well a few feet away, iii) the public well would likely be at the Wells on the common]At both Ends of the Town is Chalk dug, and the Hill here and there hath Veins of blew Loam. Of the private Well, which was newly sunk, I inform'd my self by examining the Earth cast out of it, which I receiv'd of the Owner Mr. Symonds, together with this Account.[this is a new well in the town]
The upper Earth, for two Spit deep, was the same; then they came to a harder and Loamy, which lasted about seven Feet; then to a looser, which sparkled with small Selenites, as at the publick Well; this held for two Feet, where they came at the Stones and Water together. The Water in Summertime flow'd in at the rate of an Ale-Barrel in 24 Hours. Below the Selenites, they came at a dead heavy Earth and black, partaking of Iron, under which was the common dead Loam, or Cortex of the Mineral Region: And though they dug three or four Feet deeper, yet neither was the Water, or the former Signs found. As the Selenites had somewhat of the Shape of Vitriol of Iron [ferric/ferrous sulphate], so where they lay, were Veins of Iron and Colour'd Earth; the Iron was pure, and obey'd the Loadstone; the Earth, which was either of a Brimstone Colour, or that of Iron Rust, I prov'd by washing, to be the same, only joined by an acid Juice, like Spirit of Vitriol [sulphuric acid], which in the yellow had no Taste of the Iron, but a distinct pleasant Acid; which, with the Jellying of some Parts of the Earth in Aquae Fortis, especially of the whiter Part of it, where the Selenites lay, is what I observ'd there. I shall not therefore repeat my Tryals of Earths, which were fruitless.
The Water is moderately clear, of Taste bitter, together with a maukish Saltiness, not manifestly Laxiviat, but a little of the Taste of the second Salt of Salt Marine, and of that Cellar-Salt that is gather'd by Things hanging in the Middle of Cellars, and not what fixes to the Walls."
He then described various chemical tests made to determine the nature of the salt, observing that half an ounce purged pleasantly.[12]
The land on which Symonds dug the well can be identified, through the Manorial Survey of 1697, as being that formerly of Humphrey Beane known as "Warrens".[13] This lay at the western end of Epsom High Street. The Symonds well is therefore believed to be on the Warrens, near the former Magpie Inn, now Symond's Well Restaurant. This well should not be confused with the Old Well at The Warren near the racecourse. The public well referred to by Allen would likely have been the Old Well on the common. Then there was the new Symond's Well in the town with a second well nearby.
Allen gave more detail of the Symonds well in his 1711 new edition which also discusses the public well (which we can presume to be the one on the common). Symonds well was described as being in the town and about 10 feet deep. Allen had originally inspected it two days after digging and it yielded rod-like selenites 2-3 inches long. The water oozed in at a rate of a barrel in 24 hours. Allen also mentions a well in the house next door which yielded diamond shaped crystals.[14] This was the well described in the 1699 edition as a few feet away. Lehmann (1973) suggested that Symonds was the well that Livingstone used, the well a few feet away next door however must also be a contender and this is now discussed.[15]
In 1670, Humphrey Beane bought land in Epsom which included "Warrens" believed to be about 3 acres on the Dorking Road behind the Magpie Inn. In his will, recorded at the Court Barons 1679/80 & 80/81, he left the land to John Parsons, his son-in-law. In 1701 and 1707 Livingstone had acquired land once owned by Humphrey Beane, as mentioned earlier. The latter purchase was from John Parsons. This all became the New Wells and abutted land owned by a John Symonds which was also formerly Beane's land. The Beane will, under which Parsons inherited the estate, recorded a well of water with pipes across the road to his wash house.[16] This is the original Warrens well. In addition there was the well described as Symonds well, also believed to be on what was formerly Beane's land, which was dug about 1699 and found to contain Epsom Salts.[17]
It can be argued with some confidence that these two sources are the wells mentioned by Allen. Either or both are the source that Livingstone relied on for his principal water supply. Livingstone would have been able to transport the water to his New Wells site without traversing a road, possibly by pipeline in the case of the Warrens Well. Unfortunately any claim to substantial mineralisation will now be shown to be suspect.
Reference to the 1978 Geological Survey map[18] enables a hypothesis to be constructed. Livingstone's New Wells were situated at the western end of the High Street. This area falls just within the boundary of the London Clay which overlays the Epsomite bearing strata. To the east there is a surface geology anomaly in that the recent Teale Gravels overlay the district. This anomaly is of minor significance in the following argument and is therefore ignored to all intents and purposes. Livingstone's New Wells were therefore sited to be able to secure well water charged with Epsom Salts, but only just. Inspection of the map indicates that the Old Wells and Jessop's Well, located on the London Clay, are much further from the surface boundary of the Epsomite bearing strata and historically contain more Epsom salts. Can it be argued therefore that there is a relationship between the strength of the well and the distance from the surface boundary of the Epsomite strata?
Exwood recorded a number of historic analyses of Epsom and nearby wells. The analyses have been converted to drachms/gallon, where in other units, for comparison purposes.
date author well contents
1695 Grew Old Well 6-10 drachms/gallon
1699 Allen Old Well 7 drachms/gallon
1711 Allen New Well 0.5 drachms/pint(= 4d/g)
1725 Hoffmann Epsom 0.5 quentgen/medic.pound
1751 Hales Epsom 34 grains/pound(avoir)(=4.53d/g)
Cobham 60-68 grains/pound(=8.53d/g)
Jessop's 82 grains/pound(=10.93d/g)
1756 Lucas Old Well 40 grains/pint(=5.33d/g)
12.4. Historic analyses of Epsom Salts in various wells.[19]
From the above it can be seen that Cobham and Jessop's were more substantially charged than the Epsom Wells, also that the New Well was less charged than the Old Well. Jessop's Well, which is nearby, would have therefore been a more desirable source for Epsom mineral waters than the Epsom Wells, particularly if the supply were more plentiful and reliable.
The historic analyses are now matched against the distance from the surface boundary of the Upper Chalk and the Thanet Beds. The units used are grams per litre after Exwood and kilometres. A 1989 analysis of the Old Well given by Exwood has been disregarded because the well was not adequately pumped to clear rain and surface water before testing.
Old Well (Allan) - 7.2 g/l - 1.6km.
New Well (Allen) - 4.1 g/l - 0.5km.
Epsom (New Well?) (Hales) - 4.8 g/l - 0.5km
Cobham (Hales) - 9.15 g/l - 4.1km.
Jessop's (Hales) - 11.7 g/l - 3.9km.
Old Well (Lucas) - 5.5 g/l - 1.6km
12.5. Epsom salt content of wells and distance from geological surface boundary.
In recent times the artesian mechanism has weakened in effect due to the depletion of the chalk aquifer for public water supplies. As a result any evaluation is dependent on historic analysis. The comparison between distance from the geological boundary and the concentration of Epsom Salts in the water can be graphed out as follows in 13.6. 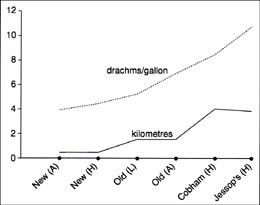 12.6. Relationship between well concentration and distance from the geological boundary. The upper line represents the concentration and the lower line the distance. A letter identifying the origin of the analysis suffixes the New and Old Wells analyses.
12.6. Relationship between well concentration and distance from the geological boundary. The upper line represents the concentration and the lower line the distance. A letter identifying the origin of the analysis suffixes the New and Old Wells analyses.
Figure 12.6 suggests that there may be a correlation between distance and salt concentration although the sample size is small. As the distance increases so does the concentration. The mechanism that brings this about results from two considerations. Firstly the recharge zone of the Epsomite bearing strata lies where the beds surface, drawing water from the chalk springs along this juncture. Secondly, the further the well is from the juncture, the longer the distance the water travels through the beds and the greater the solution time for the absorption of Epsom Salts. Indications from early literature suggest that the movement of water through the Epsomite aquifer is slow, thereby accounting for the rapid depletion of well contents and the need to replenish the exploited wells, possibly from other mineralised wells nearby.
What is apparent is that Livingstone's New Wells were the weakest of the celebrated local wells and this would cause problems. Firstly he would have been short of the essential mineralised water, if his well flowed at similar rates to that of the Old Well. Every morning the supply of impregnated water, collected overnight, would have been quickly utilised. Secondly, the water that he did secure through natural causes would have been lower in concentration of Epsom Salts and therefore less effective, requiring larger doses. This would have put further demand on his supply.
The implications of this are considered with the benefit of hindsight and enhanced scientific knowledge in the ensuing chapters.
Click on website below to return to Index and Introduction.
Website: Click Here
SUPPLEMENTARY INFORMATION
1) TOPOGRAPHICAL LOCATION:
England
3) INFORMATION CATEGORY:
Springs and Wells General InterestHistory & Heritage
Friends Newsletter


